Plastics are stronger and lighter thanks to carbon nanotubes derivatives
30.09.2024
 |
|
Test tube for mechanical testing. Credit: Ion Isasti. |
- IMDEA Nanociencia researchers reinforce a type of plastics with derivatives of carbon nanotubes.
- These reinforced plastics are entirely recyclable, they can be melted down to repair or reshaped into other objects, keeping their properties intact.
- This work is being done in collaboration with the Danish innovation company Nanocore.
- Reinforced plastics are strong and lightweight, and contribute to more sustainable production.
| Tweet |
Madrid, 30th September, 2024. Reducing the environmental impact caused by plastics can be addressed through different strategies, such as the manufacture of more durable plastics or recycling. In general, there are two main types of plastics. The first is thermoplastics, which can be melted and molded to form other objects, although their mechanical properties weaken if they are melted several times. And the second, thermosets, do not melt at high temperatures, since the chains of the polymers that form them are intertwined by chemical bonds.
Thermoset plastics have advantageous properties compared to thermoplastics. They tend to have a higher resistance to impact and mechanical stress, although they are also more brittle. Epoxy resin, silicone or melamine are examples of thermoset plastics, commonly used in construction. To make these plastics stronger, engineers add reinforcement materials such as carbon fibers. They are already used to manufacture objects such as motorcycle helmets or sports equipment, which are very durable although they cannot be easily recycled.
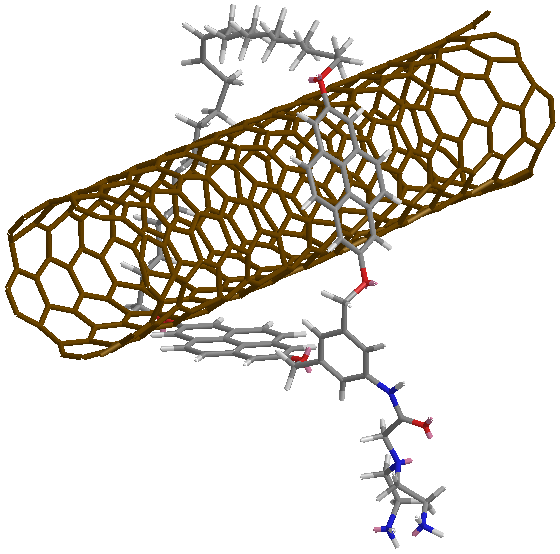
 At IMDEA Nanociencia, the Chemistry of Low-Dimensional Materials group, led by Emilio Pérez, is investigating a strategy to strengthen recyclable plastics in a collaboration with the company Nanocore. The plastic studied is a 'covalent adaptable network', whose molecular structure is similar to that of a thermoset plastic but with the particularity that it incorporates covalent– strong – bonds but at the same time reversible between polymer chains. Specifically, they work with imines, whose bonds are dynamic: can be broken by water or temperature, and re-arrange. The novelty of the study lies in the use of a derivative of carbon nanotubes that have a ring molecule around them - mechanically interlocked carbon nanotubes MINTs. The ring molecules are attached to the carbon nanotube mechanically, not chemically, so the bond between the two is very strong, but at the same time allows a certain movement of the molecule along the nanotube. The researchers have equipped the ring with two anchor points (two amines) so that they covalently bond with the polymers. In this way, the nanotube becomes a structural part of the polymer network.
At IMDEA Nanociencia, the Chemistry of Low-Dimensional Materials group, led by Emilio Pérez, is investigating a strategy to strengthen recyclable plastics in a collaboration with the company Nanocore. The plastic studied is a 'covalent adaptable network', whose molecular structure is similar to that of a thermoset plastic but with the particularity that it incorporates covalent– strong – bonds but at the same time reversible between polymer chains. Specifically, they work with imines, whose bonds are dynamic: can be broken by water or temperature, and re-arrange. The novelty of the study lies in the use of a derivative of carbon nanotubes that have a ring molecule around them - mechanically interlocked carbon nanotubes MINTs. The ring molecules are attached to the carbon nanotube mechanically, not chemically, so the bond between the two is very strong, but at the same time allows a certain movement of the molecule along the nanotube. The researchers have equipped the ring with two anchor points (two amines) so that they covalently bond with the polymers. In this way, the nanotube becomes a structural part of the polymer network.
Putting rings on nanotubes: a simple and very effective strategy
Carbon nanotubes are essentially a sheet of graphene rolled up on itself. To join a nanotube with other molecules, it is possible to do so directly by covalent bonds which break the tube a little, add defects and weaken it. The strategy pursued by the researchers uses the mechanical bond – a ring molecule around the nanotube – to integrate the nanotubes into the polymer lattice, preserving all their properties, and maximizing the load transfer from the matrix to the reinforcement. In other words, it cannot be done better.
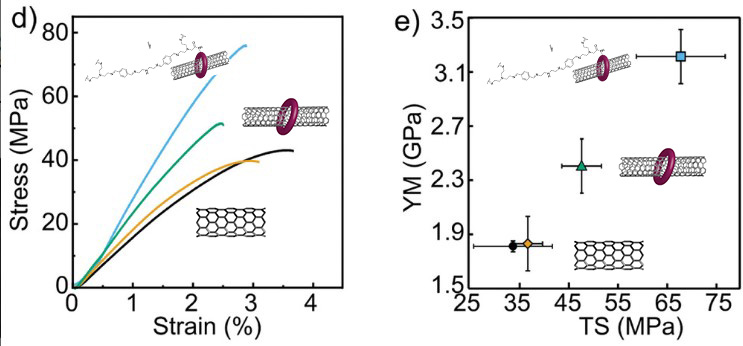
 The concept is simple: by surrounding the nanotube with a ring, the agglomeration of these fibers that makes the reinforcement less effective is prevented. In addition, polymer interaction sites are provided in the ring, which improves stress transfer. Adding only 1% nanotubes by weight to the polymer mixture achieves a 77% improvement in Young's modulus, and a 100% improvement in tensile strength. Remarkably, the mechanical properties of this reinforced plastic remain intact after being melted down and recycled up to 4 times.
The concept is simple: by surrounding the nanotube with a ring, the agglomeration of these fibers that makes the reinforcement less effective is prevented. In addition, polymer interaction sites are provided in the ring, which improves stress transfer. Adding only 1% nanotubes by weight to the polymer mixture achieves a 77% improvement in Young's modulus, and a 100% improvement in tensile strength. Remarkably, the mechanical properties of this reinforced plastic remain intact after being melted down and recycled up to 4 times.
The mechanical properties of this reinforced plastic remain intact after being melted down and recycled up to 4 times.
In engineering, the Law of Mixtures indicates that the properties of a compound are the mixture of the properties of the original materials, according to their proportion. The study led by the Madrid researchers confirms that this is only the case when there is an efficient transfer of mechanical stress between both compounds, at the nanoscopic level. In their work, the researchers have achieved maximum efficiency in transferring mechanical stress from the polymer to nanotubes, the strongest material. Nanotubes have a Young's modulus of 1TPa, 5 times harder than steel, being a much lighter material. Adding more nanotubes to the plastic does not make it stronger, as the nanotubes begin to agglomerate and lose efficiency. The key to success lies in the covalent bond between the nanotubes and the polymer.
It all started with an ERC grant
The story of this scientific result begins in 2012, when researcher Emilio Pérez received a prestigious 'Starting' grant from the European Research Council (ERC) to develop an innovative idea: putting molecular rings on carbon nanotubes. With this grant of 1.5 million euros, Pérez consolidated his research group at IMDEA Nanociencia institute. For 5 years, they created mechanical bonds between ring molecules and carbon nanotubes and studied their properties, although without yet focusing on their possible applications. In 2017, Pérez received a call from Nanocore ApS, a Danish company pioneering the transfer of results to the materials production market. They also intended to modify carbon nanotubes, from a biochemical approach. They began collaborating together on a year-long project, and then obtained an ERC 'Proof of Concept' grant that promoted the experiments. In 2020 they would sign a contract of more than 3 million euros to work together on the reinforcement of plastics with carbon nanotubes.
A myriad of applications
The collaboration with Nanocore continues to explore all the most commercially relevant polymers that can be reinforced. Producing plastics almost as strong as carbon fibers, which can be melted down and recycled, is a dream. A before and after, which can firmly contribute to a new, greener and more sustainable scenario. Pérez explains: “Producing lighter structures, such as cars, planes, etc., would mean considerable fuel savings.” Manufacturing with less material and ensuring recyclability draws a promising horizon.
This work has been carried out at IMDEA Nanociencia and is partially funded by the Severo Ochoa Seal of Excellence awarded to IMDEA Nanociencia (CEX2020-001039-S).
Glossary:
- Carbon fiber: synthetic filaments of microscopic diameter, made up of well-packed graphene sheets.
- Carbon nanotube: rolled sheet of graphene shaped as a tube of carbon atoms.
- Mechanically interlocked nanotubes, MINT: nanotubes that have a ring molecule around them, mechanically (not chemically) bonded.
- Imine: organic compound containing a carbon-nitrogen double bond.
- Young's modulus: parameter that characterizes the elasticity of a material, and determines the deformation as a function of the applied stress.
Reference:
I. Isasti, S. Miranda, D. M. Jiménez, S. Parzyszek, N. Martín Sabanés, H. Pedersen, E. M. Pérez, Reinforcement of polyimine covalent adaptable networks with mechanically interlocked derivatives of SWNTs. Adv. Funct. Mater. 2024, 2408592. https://doi.org/10.1002/adfm.202408592
![]() https://repositorio.imdeananociencia.org/handle/20.500.12614/3757
https://repositorio.imdeananociencia.org/handle/20.500.12614/3757
Contact:
Prof. Emilio M. Pérez
emilio.perez (at)imdea.org
Chemistry of Low Dimensional Materials Group
https://nanociencia.imdea.org/chemistry-of-low-dimensional-materials/home
@emiliomperezlab
Oficina de Divulgación y Comunicación en IMDEA Nanociencia
divulgacion.nanociencia [at]imdea.org
Twitter: @imdea_nano
Facebook: @imdeananociencia
Instagram: @imdeananociencia
Source: IMDEA Nanociencia.
IMDEA Nanociencia Institute is a young interdisciplinary research Centre in Madrid (Spain) dedicated to the exploration of nanoscience and the development of applications of nanotechnology in connection with innovative industries.
Related information:
Proyecto Tsunami: la investigación que tiene entre manos el material del futuro https://www.innovaspain.com/imdea-nanociencia-nanotubos-carbono-emilio-perez-nuevos-materiales/
Mechanically interlocked derivatives of carbon nanotubes: synthesis and potential applications https://pubs.rsc.org/en/content/articlehtml/2022/cs/d2cs00510g




