Playing with the weakest supramolecular interactions in a fullerene compound
09.09.2021
 |
|
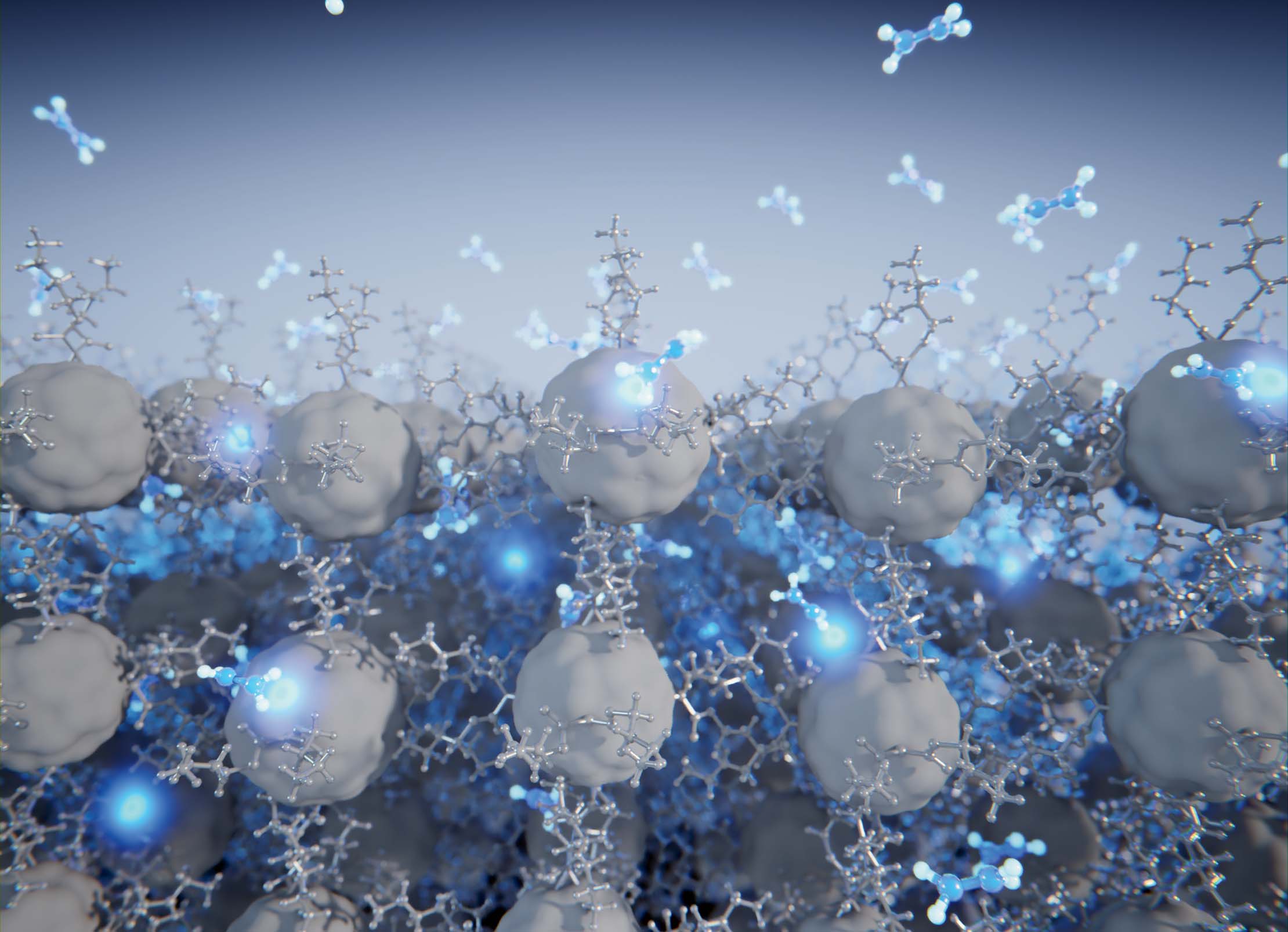
Controlled reorganization of a fullerene-based compound (grey), purely governed by the weakest van der Waals interactions (dihydrogen) and the entry of small molecules into its interior (hydrazine molecules in blue). Image: Scixel. |
- Researchers at IMDEA Nanociencia control the weakest van der Waals forces in a fullerene C60 compound by applying an external stimulus.
- The study represents a landmark achievement in the field of Supramolecular Chemistry, where weak forces can play a crucial role in chemical reactions.
- This strategy has allowed for the first time to selectively and in a controlled manner hydrogenate the C60 and may have relevant implications for hydrogen storage.
Madrid, September 9th, 2021. In a chemical reaction, the bonds that stick atoms to each other break and set up to form new compounds. Apart from ionic or covalent bonds, there are also other types of interactions --the weak van der Waals forces-- which can play an essential role in chemical reactions, in the speed or selectivity of reactions. Controlling these subtle forces in the processes of molecular reorganization through the application of a thermal or chemical stimulus is one of the long pursued goals of Supramolecular Chemistry.
In a study published in the journal Chemical Science, researchers from IMDEA Nanociencia and the University of Barcelona, led by Dr. José Sanchez Costa, propose a novel synthesis strategy based on the controlled reorganization of a fullerene compound, purely governed by the weakest van der Waals forces: the dihydrogen bond.
The authors highlight two outstanding features that make their work particularly original. First, it significantly advances knowledge about dihydrogen binding, also called "sticky fingers", a type of cumulative interactions found in nature that are responsible for, for example, geckos climbing walls or interactions in cells lipid layers. Secondly, the transformation of the material as a whole is controllable. The application of an external perturbation allows to modify the character of the interactions and to switch the system from one phase to another. Each phase of the material interacts in different ways with external molecules that pass through the "sticky fingers" and give rise to different reactions within the same material.
Specifically, supramolecular reorganization was selectively controlled by an external stimulus: exposure of the crystal to hydrazine vapors (N2H4). Depending on the material’s phase, hydrazine hydrogens the C60 ball to a greater or lesser extent. In addition, the monitoring of the reaction through single crystal diffraction allowed the direct observation of molecular movements within the network and the subsequent hydrogenation reaction. This reaction is also accompanied by a colour change of the system from orange to yellow.
“These materials could have potential application in hydrogen storage.”
Separating the different key supramolecular contributions to the stability of 3D systems is still a major challenge. Therefore, the authors' ability to dictate reorganization in a controlled manner, separating supramolecular contributions, satisfies one of the main targets of Supramolecular Chemistry, and helps to understand the intrinsic nature as well as the chemical reactivity of supramolecular ensembles.
The study is a result of the "Switchable Nanomaterials" group at IMDEA Nanociencia, led by Dr. José Sánchez Costa, in collaboration with the group of Prof. Nazario Martín at the Complutense University of Madrid, and Carolina Sañudo (University of Barcelona), and has been cofunded by the Severo Ochoa Centre of Excellence seal granted to IMDEA Nanociencia in 2017.
Reference:
E. Fernández-Bartolomé, A. Gamonal et al. Playing with the weakest supramolecular interactions in a 3D crystalline hexakis[60]fullerene induces control over hydrogenation selectivity. Chem. Sci. 2021.
DOI: 10.1039/D1SC00981H
Contact
Dr. José Sánchez Costa
jose.sanchezcosta [at]imdea.org
https://nanociencia.imdea.org/home-en/people/item/jose-sanchez-costa
Twitter: @josescostalab
Oficina de Comunicación de IMDEA Nanociencia
divulgacion.nanociencia [at]imdea.org
Twitter: @imdea_nano
Facebook: @imdeananociencia
Instagram: @imdeananociencia
Source: IMDEA Nanociencia





